Introduction: Irradiation of blood products is necessary to prevent transfusion-associated graft-versus-host disease (TA-GVHD) for patients at risk of this fatal transfusion complication. Despite the implementation of protocols to identify patients at risk of TA-GVHD, missed irradiation events still occur; therefore, some institutions have adopted a policy of universal irradiation of blood products to prioritize safety. Universal irradiation, however, could have an adverse impact on transfusion outcomes as red blood cell (RBC) unit irradiation has been associated with smaller hemoglobin (Hb) increments, biological changes to the RBC membrane, shortened RBC survival, and potassium release. These potential adverse effects of irradiation may be especially important for patients with sickle cell disease (SCD) receiving chronic transfusion, a group that does not require irradiated blood products.
Methods: Our pediatric institution implemented a policy of universal blood product irradiation in May 2018 in which all RBC units are irradiated typically at time of issue. We conducted a retrospective chart review of patients with SCD receiving simple chronic RBC transfusion throughout the year before (5/3/2017-5/3/2018) and the year after (5/4/2018-5/4/2019) this policy change. Patients were excluded if during either time period they received fewer than 10 transfusions, chronic transfusion targets were modified, hydroxyurea was started, they received more than 5 RBC units for an acute complication, or they were transfused at an outside institution. The primary outcome was the change in RBC transfusion volume per patient weight transfused during the pre- vs. post- universal irradiation time period. Secondary outcomes were the change in median pretransfusion laboratory values. The change in these outcomes was analyzed with the Wilcoxon matched-pairs signed rank test.
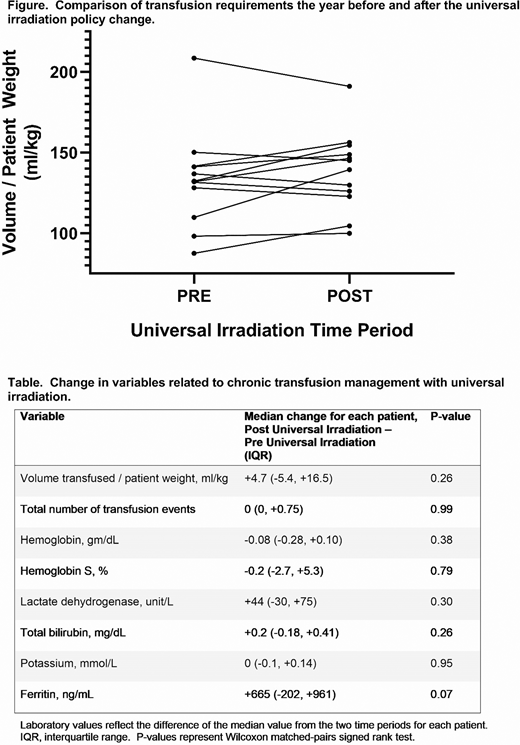
Results: Among 59 chronic transfusion patients, we identified 12 who fit our inclusion/exclusion criteria (5 males, 7 females), all of whom were on chronic transfusion for either primary or secondary stroke prevention. Regarding SCD genotype, 10 patients had Hb SS, and 1 patient had Hb Sβ0 thalassemia. The median patient age at the time of the irradiation policy change was 10 years (range 3 to 18 years). During the pre- universal irradiation time period only 4% (10/230) of units transfused to these patients were irradiated, likely due to reallocation from other patients. As expected per the policy change, 100% (271/271) of units were irradiated in the universal irradiation time period. During both time periods, patients received a median of 12 transfusions. When comparing the volume transfused per patient weight during these two time periods for each patient, 5 patients (42%) received more RBCs the year prior to universal irradiation and 7 (58%) received more RBCs in the year following the policy change (Figure). For the entire group, the median difference in volume per patient weight was +4.7 ml/kg, p=0.26. When comparing the change in median pretransfusion Hb, Hb S, lactate dehydrogenase, total bilirubin, potassium, and ferritin, there were no significant differences (Table).
Conclusions: In a cohort of patients with SCD receiving simple chronic transfusion for stroke prevention, a new policy of universal irradiation did not impact transfusion requirements or pertinent pretransfusion laboratory values. Universal irradiation does not appear to have clinically significant consequences for SCD chronic transfusion management.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal